Abstract
Background: Fms-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD) AML is associated with an increased risk of relapse, leading many patients to receive an allogeneic hematopoietic stem cell transplantation (HCT) after induction therapy. Unfortunately, relapse after HCT remain high and strategies are needed to improve outcomes. The SORMAIN trial has demonstrated survival benefit with sorafenib (SORA) maintenance after HCT in FLT3-ITD AML.
Gilteritinib (GILT) is a second generation FLT3 inhibitor with efficacy in FLT3-ITD AML and higher FLT3 potency compared to other FLT3 inhibitors. Our institution utilizes GILT and SORA, per physician's preference, as post-HCT maintenance in all FLT3-ITD AML. In this study, we aim to compare efficacy and safety between GILT and SORA as post-HCT maintenance.
Methods: We performed a retrospective analysis of adult patients with FLT3-ITD AML who received allogenic HCT from 6/1/2016 to 12/31/2020 and thereafter received SORA or GILT as post-HCT maintenance (off clinical trial). Patients included were in complete remission (CR) after HCT at the time of initiation. Primary outcome was 1-year progression-free survival (PFS) after initiation of maintenance. Secondary outcomes included overall survival (OS), non-relapse mortality (NRM), relapse, and adverse events (AE) 1 year after maintenance was started. AEs observed were collected retrospectively by chart review and classified according to CTCAE criteria.
Results: A total of 55 patients were treated with either GILT (n=27) or SORA (n=29) for post-HCT maintenance. One patient was treated with SORA after first HCT and GILT after second HCT. Patient characteristics, as presented in Table 1, were comparable between groups. FLT3 inhibitors were utilized in pre-HCT therapy in all but 1 patient.
Median time to initiation of GILT was 60 days (range, 21-233) after HCT and the most common starting dose was 40 mg/day. Median duration of time that patients remained on GILT was 385 days (range, 10-804). Median time to start SORA was 58 days (range, 31-191) after HCT and the most common starting dose was 200 mg/day. Median duration of time that patients remained on SORA was 315 days (range, 3-1777).
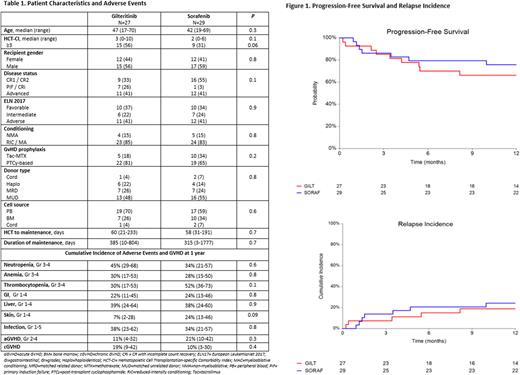
Median follow-up time of survivors was 16 and 33 months in the GILT and SORA groups respectively. As shown in Figure 1, 1-year PFS and relapse incidence were similar between GILT and SORA; PFS was 66% vs. 76% (p=0.4) and relapse incidence was 19% vs. 24% (p=0.6), respectively. OS at 1 year, 78% vs. 83% (p=0.4), was also comparable. However, NRM at 1 year was higher in the GILT group, 15% vs 0% in the SORA group (p=0.03). Causes of death in the GILT group (n=4) included 1 diffuse alveolar hemorrhage and 3 pneumonias (2 viral and 1 interstitial).
As presented in Table 1, both groups had high incidence of Grade 3-4 neutropenia, anemia, and thrombocytopenia. This was followed by liver toxicity (39% vs. 38% in SORA and GILT groups) and infections (38% in both groups). As expected, there was higher incidence of skin toxicity in the SORA group compared with GILT (28% vs. 7%, p=0.09). GVHD rates appeared comparable. Although the intention was to give maintenance for 1 year, 13 patients (48%) in the GILT group and 15 patients (52%) in the SORA group discontinued maintenance before completion of 1 year. Reasons included relapse (n=5), cytopenias (n=2), pulmonary toxicity (n=2), cardiac toxicity (n=1), GI toxicity (n=1), eosinophilia (n=1), and viral infection (n=1) in the GILT group and relapse (n=6), cytopenias (n=6), skin toxicity (n=2), and liver toxicity (n=1) in the SORA group. Four patients switched from SORA to GILT but none switched from GILT to SORA.
Conclusions: Our results revealed comparable PFS, OS, and relapse incidence when SORA and GILT are used as post-HCT maintenance therapy. Both drugs were associated with high incidence of grade 3-4 cytopenias. Despite similar toxicity profile with GILT and SORA, 1-year NRM in the GILT group was higher than the SORA group. Higher incidence of HCT-CI ≥ 3 in the GILT group may have contributed to this difference. Since our analysis was retrospective and AEs were not collected in real time, the attribution of observed AEs to the maintenance drug could not be assessed and captured. Therefore, we cannot provide an explanation of the higher NRM after GILT despite similar documented AEs. We await the results from BMTCTN 1506 that would provide more insight to the efficacy and tolerability of GILT in the post-HCT setting.
Disclosures
Yeh:Omeros: Honoraria. Ahmed:Xencor: Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Chimagen: Consultancy, Research Funding; Seagen: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Consultancy; Merck: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Mehta:Orca Bio: Research Funding; Syndax: Research Funding. Popat:Bayer: Research Funding; Iovance: Consultancy; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Champlin:General Oncology: Other: Data Safety Monitoring Board; Johnson &Johnson: Consultancy; Bluebird: Other: Data Safety Monitoring Board; Cell Source Inc.: Research Funding; Kadmon: Consultancy; Omeros: Consultancy; Actinium: Consultancy. Shpall:Takeda: Patents & Royalties; axio: Consultancy; NY blood center: Consultancy; Fibroblasts and FibroBiologics: Consultancy; adaptimmune: Consultancy; Bayer: Honoraria; Navan: Consultancy; Affimed: Other: License agreement. Oran:AROG: Research Funding; ASTEX: Research Funding.
OffLabel Disclosure:
Gilteritinib and Sorafenib
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal